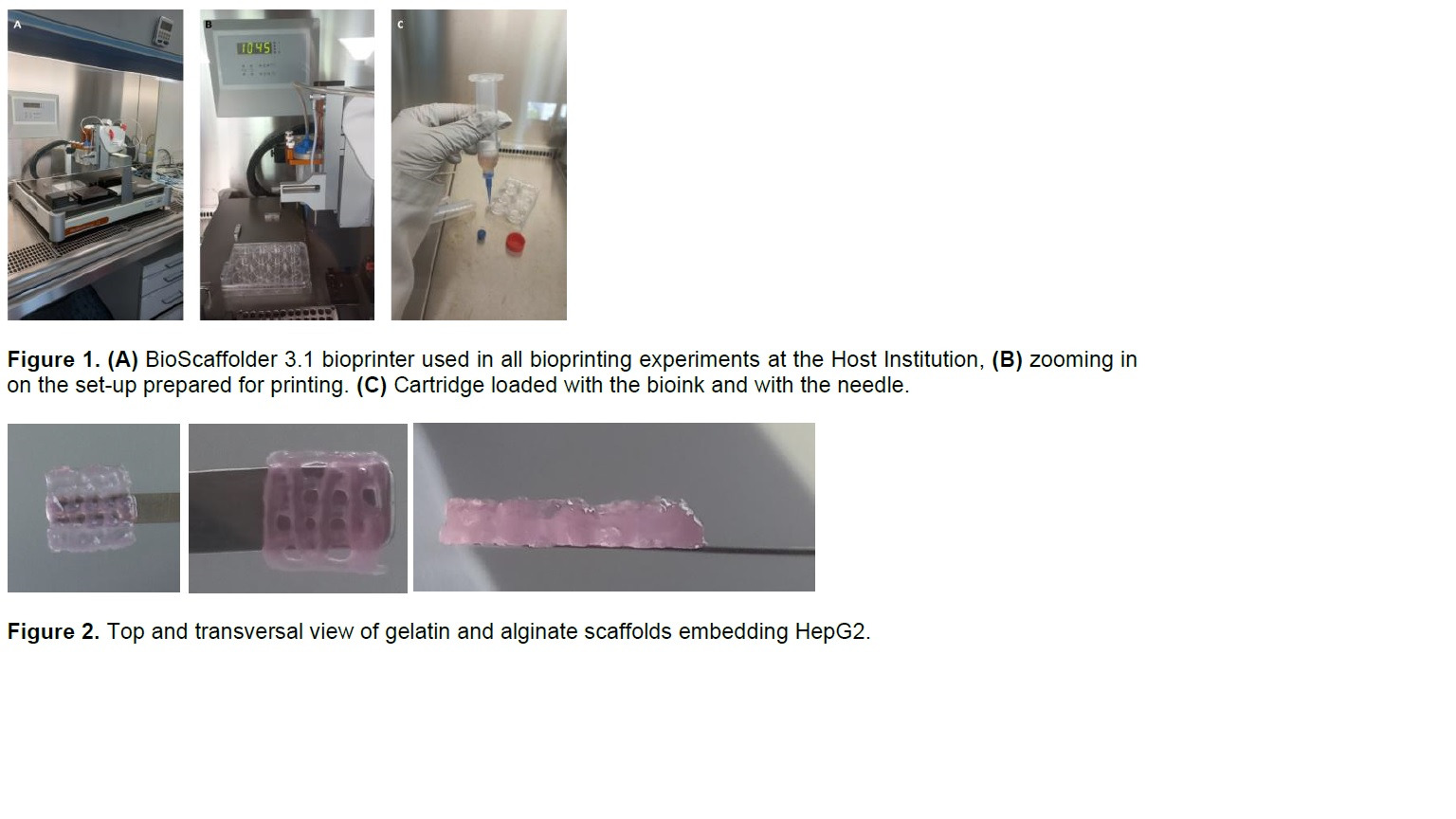
Through this STSM, the applicant aimed at learning the methods available at the Host Institution for the bioprinting of alginate-based bioinks embedding hepatic cells, as well as techniques for evaluating the bioprinted constructs and the cells embedded in them. Briefly, during the STSM, the applicant had opportunity to perform the bioprinting of alginate and gelatin bioinks and test the effect of different crosslinking conditions on the stability of the bioprinted constructs. The referred bioinks were also used for embedding hepatic cells, mainly hepatocytes (HepG2 cell line) and hepatic stellate cells (LX-2 cell line). Cells embedded in the scaffolds were analysed using different techniques. Moreover, the applicant was also able to test the 3D bioprinting of a different bioink composed by collagen type I and alginate. The system used for bioprinting at the Host Institution is presented in Figure 1, and representative pictures of the printed scaffolds are shown in Figure 2.
Overall, the initial work plan proposed in the STSM was achieved, as the applicant was able to gain extensive hands-on experience on 3D bioprinting of alginate-based bioinks for creating in vitro liver models that will be essential for her work at the Home Institution iS3 in Porto, Portugal.
Within the STSM which took place at the IRCCS Fondazione Policlinico Universitario A. Gemelli from the 5th of April until the 5th of June 2024, I took a 2-month hands-on-learning visit at the Organoids Research Core Facility under the supervision of Professor Claudio Sette.
I learned a lot about the culture and handling of high-grade serous ovarian cancer organoids and ovarian cancer cell lines, as well as different advanced experimental techniques such as western-blot, proliferation and viability assays.
I have had the opportunity to advance the research associated with my PhD project, being able to acquire valuable data and results that will undoubtably enhance the potential of the compound we are developing.
Moreover, this STSM also contributed to the advance my academic and professional growth.
I can say with certainty that I have broadened my knowledge about in vitro 3D models, particularly organoids.
The visit to IRCCS Fondazione Policlinico Universitario A. Gemelli was an extremely pleasant experience and I would not hesitate to do it again.

Figure: Three different high-grade serous ovarian cancer organoids cultured and handled during my experience.
I am Paolo Signorello, a PhD student at the Department of Information Engineering, University of Pisa, and a member of Centro 3R, Italy. Thanks to the Short-Term Scientific Mission (STSM), I spent four months (from 01/02/2024 to 31/05/2024) at “i3S - Instituto de Investigação e Inovação em Saúde da Universidade do Porto”, under the supervision of Professor Bruno Sarmento. During this period, I developed a magnetic nanoparticle-based formulation and tested it in an advanced in vitro intestinal model1 for potential use in drug delivery or hyperthermic therapy targeting the intestine.

This model consisted, from top to bottom, of a multilayer co-culture using transwells for 24-well plates, which included four different cell types: two types for the epithelial layer, fibroblasts encapsulated in a hydrogel to ensure the development of the extracellular matrix in the apical compartment, and endothelial cells in the basolateral compartment. This configuration was designed to mimic the complex structure of the intestinal barrier.
First, I functionalized magnetic nanoparticles with a mucoadhesive biological material and tested them for stability over time and at different pH levels using a Dynamic Light Scattering (DLS) instrument.
Subsequently, I conducted a viability assay to evaluate the toxicity of the magnetic nanoparticles on all cell types. After 21 days of developing the model, I tested for any biological differences that may occur in terms of permeability and nutrient absorption, both in the presence and absence of the magnetic nanoparticle-based formulation.
I am very grateful to the COST Action IMPROVE for supporting this period abroad at i3S, as it significantly enhanced my knowledge of nanomaterials, cytotoxicity, the development of complex in vitro models, and co-culture techniques.
The atmosphere at i3S is very welcoming and inclusive. Furthermore, Professor Sarmento’s team is very proactive in building new working groups and fostering networking opportunities. This experience will allow me to engineer better, more advanced models with improved predictivity, in line with the 3Rs Principles.

[1] Ferreira B, Barros AS, Leite-Pereira C, Viegas J, das Neves J, Nunes R, Sarmento B. Trends in 3D models of inflammatory bowel disease. Biochim Biophys Acta Mol Basis Dis. 2024 Mar;1870(3):167042. doi: 10.1016/j.bbadis.2024.167042. Epub 2024 Jan 29. PMID: 38296115.
Within the STSM which took place at Swansea University Medical School from the 15th of April until the 19th of April 2024, I took an intensive 4–5-day hands-on-learning visit at the Department of the In Vitro Toxicology Group under the supervision of Professor Shareen Doak.
I learned a lot about the handling of nanomaterials in the field of in vitro toxicology, different kinds of exposure techniques of advanced (3D) in vitro cell models, techniques for studying nanomaterial cell internalization, and was introduced to the approach they use for assessing the nanoparticle toxicity.
I have successfully learned about the safe handling of nanomaterials (especially when it comes to dealing with waste that contains nanomaterials), I have seen different kinds of exposure techniques for both 2D and advanced 3D cell models and gotten to know the method used for nanomaterial cell internalization (TEM).
I have had a chance to see and try two additional methods for preparing spheroids not used at our institute – the Institute of Biology, Ljubljana, Slovenia.
What is even more important I have learned the comprehensive approach of nanoparticle toxicity assessment from preparing the suspension of nanoparticles, assessing their characteristic, and performing sonication, to exposing 2D and 3D cell models to nanoparticles using different exposure systems.
I can say with certainty that I have broadened my knowledge about nanomaterial handling, nanosafety, and different approaches in toxicology studies for assessing nanomaterial-based toxicity on 2D and advanced in vitro models. The visit to Swansea University was an extremely pleasant experience and I would not hesitate to do it again.


Dr. Eva Jablonská (University of Chemistry and Technology in Prague, Czech Republic) had a great opportunity to spend more than two months (15.1.-22.3.2024) at the Dermatotoxicology Study Centre, German Federal Institute for Risk Assessment (BfR) in Berlin. During the STSM granted by CA IMPROVE, she tested natural extracts from marine algae for photoprotective properties. Specifically, she irradiated human dermal fibroblasts with UV light and tested whether these extracts could prevent cellular damage.
Overall, the atmosphere at BfR was very welcoming and supportive. Additionally, the discussion about methods of genotoxicity testing were very fruitful.
Moreover, the grantee was fortunate to attend a three-day symposium on genotoxicity held by BfR, where quantitative approaches towards genotoxicity assessment were discussed.

